How to choose the right device for the severely calcified lesion
Christopher KOO, Huay Cheem Tan
National University Heart Centre, Singapore
Within routine cardiac catheterisation, the prevalence of calcified coronary arteries has been increasing. This is even despite coronary artery calcification being frequently underestimated by angiography. Intravascular ultrasound imaging revealed that calcification was underdiagnosed in nearly half of all cases if only angiography alone was performed, and that nearly 75% of all lesions had coronary arterial calcification. (1) Severe calcification has been reported to be present in up to 20% of all cases, and is associated with lower procedural success and an increased risk of adverse cardiovascular events. (2,3)
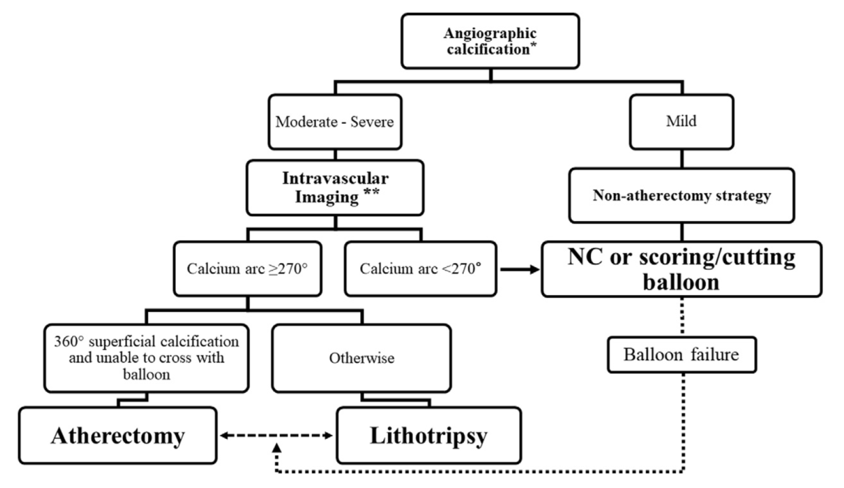
Lesion preparation of calcified arteries can be performed with cutting and scoring balloons. However, severely calcified lesions may be refractory despite repeated attempts at angioplasty. Hence, other adjunctive devices are required to tackle such lesions. Atherectomy is one such modality, and is performed with the goal of lesion preparation via plaque modification. Figure 1 is a suggested treatment algorithm for calcified vessels. (4) There are four main techniques to deal with severe coronary arterial calcification – rotational atherectomy, orbital atherectomy, intravascular lithotripsy, and excimer laser coronary atherectomy.
Rotational atherectomy
Percutaneous transluminal rotational atherectomy is used contemporarily for plaque modification. This is commonly performed with the Rotablator® system (Boston Scientific). The mechanism behind rotational atherectomy is through differential cutting. This allows for mechanical ablation of inelastic fibrocalcific plaques, and spares normal elastic tissue which deflects away from the ablating burr. This smoothens the vessel lumen, and facilitates subsequent balloon dilatation and stent implantation.
Rotational atherectomy can be performed either via radial or femoral arterial access. Although the required sheath size ranges from 6 Fr to 8 Fr depending on burr size, most procedures can be performed with a 6 Fr guide catheter which can accommodate burrs up to 1.75mm. There may be difficulties using the rotawire as a standalone coronary guidewire. Hence, most operators use a regular guidewire to advance through the complex coronary artery anatomy before switching to the rotawire using a microcatheter. Rotablation is usually performed with the floppy rotawire, although aorto-ostial lesions may specifically benefit from using the extra-support rotawire. (5) A small burr size should be used for very tortuous anatomy and long lesions, and only in aorto-ostial lesions or in vessels with a large minimal lumen diameter should larger burr sizes be considered. A single 1.5mm burr (with an approximate burr-to-artery ratio of 0.6) is generally able to achieve adequate plaque modification most of the time. (5) Several procedural techniques are important in reducing the risk of complications. These include adopting a “pecking” motion of the burr, avoiding deceleration of the burr by speeds greater than 5,000 rpm, with short duration of individual runs no longer than 30 seconds, and maintaining a safe speed of rotablation between 135,000 to 180,000 rpm. (5) The burr should be downsized if the lesion cannot be crossed after several passes. Rotational atherectomy should be stopped when there has been adequate plaque modification. Residual dog-boning during low pressure balloon angioplasty implies insufficient plaque modification and warrants further rotablation with a larger burr.
Rotational atherectomy has been performed in ostial lesions including unprotected left main coronary stenosis. Rotational atherectomy of ostial side branches before stenting of the main vessel has been reported to result in a lower rate of acute side branch occlusion. (6) A cautious step-up approach starting with a 1.25mm burr is recommended in such cases. (5) Rotational atherectomy with a 1.25mm burr may also be a useful adjunct for chronic total occlusions where the balloon has failed to cross the lesion. (5) In the hands of highly experienced operators, rotational atherectomy has also been applied to suboptimally expanded stents. (5) The benefits of rotational atherectomy followed by drug eluting stenting are decreased rates of in-stent restenosis and target lesion revascularization. (5)
Rotational atherectomy is best suited for very tight stenoses, diffuse stenosis, or vessels of small caliber. Rotational atherectomy should be avoided in angulated lesions, or large calcified lesions with a minimal lumen diameter greater than 2mm.
Orbital atherectomy
Orbital atherectomy is performed with the Diamondback 360° Coronary Orbital Atherectomy System (OAS) (Cardiovascular Systems Inc., St. Paul, MN, USA). The device comprises a 1.25mm diamond-coated crown eccentrically mounted on a ViperWireTM guidewire. The device is able to ablate calcium at low speeds of 80,000 rpm or high speeds of 120,000rpm. Through centrifugal forces, plaque modification is achieved by differential sanding. Hard non-compliant calcium is ablated by the crown, and normal healthy tissue is deflected away.
Due to the smaller debris size of less than 2 microns achieved and with continuous blood flow maintained during ablation, orbital atherectomy has been associated with a lower risk of heart block and no-reflow phenomenon. (5). Although there are no large-scale multi-centered randomized trials comparing orbital atherectomy and rotational atherectomy, the ORBIT II trial demonstrated durable benefits of orbital atherectomy with a three-year target lesion revascularization rate of 7.8% in patients with severe coronary artery calcification. (7)
The advantages of orbital atherectomy include the ability to treat large vessel size without upsizing the crown, an easier set up compared to rotational atherectomy, and a lower risk of burr entrapment. Orbital atherectomy is best suited for discrete large vessel stenosis, eccentric lesions, or lesions with a thick calcium cap. It is not as ideal for use in tortuous vessels, ostial disease, in-stent restenosis, very tight stenosis or chronic total occlusions. The system is not compatible with a 5 Fr guide, and care must be taken to avoid high speeds in small vessels to avoid perforation.
Intravascular lithotripsy
Intravascular lithotripsy is a novel balloon catheter-based device for treating calcified coronary arteries. The Shockwave Medical Coronary Rx Intravascular Lithotripsy System (Shockwave Medical Inc., Fremont, CA, USA) comprises of a single-use, disposable balloon angioplasty catheter containing a series of unfocused, electrohydraulic lithotripsy emitters. Through conversion of electrical energy into transient acoustic circumferential pressure pulses, these emitters disrupt both superficial and deep calcium. The balloon is limited to diameters of 2.5mm to 4mm and only 12mm in length. (8) The balloon should be sized 1:1 to the reference artery, and first inflated to low pressure (4 atm) delivering 10 pulses of ultrasound energy over 10 seconds per balloon. Further dilatation with a non-compliant balloon is performed, before inflation of the shockwave balloon to the size of the reference vessel. A further minimum of 20 pulses is delivered to the target lesion, with interval deflation to allow for distal perfusion. (8) A single-arm study to evaluate the safety and efficacy of intravascular lithotripsy, DISRUPT CAD I, demonstrated a low 30-day rate of adverse cardiac events of 5%, and a high clinical success rate of 95% after stenting. (9)
The advantage of intravascular lithotripsy over conventional atherectomy is achieved via circumferential plaque modification. In comparison with conventional atherectomy which is only able to modify superficial calcium, the emitted pulses from intravascular lithotripsy is able to modify both superficial and deep calcium. The large calcium fragments generated by lithotripsy also remain in-situ instead of embolizing distally, resulting in lower incidences of no-reflow syndrome. Other advantages include a lower risk of dissection, and a shorter learning curve. However, intravascular lithotripsy is limited by a high-profile delivery system, and a lack of long-term data on efficacy available currently.
Intravascular lithotripsy is best suited for discrete angulated stenosis (such as the ostial circumflex artery), calcified in-stent restenosis, or non-acute unexpanded stents. Intravascular lithotripsy should be avoided in very tight stenosis, aorto-ostial lesions, or lesions with a thick calcium cap.
Excimer coronary laser atherectomy
The use of excimer coronary laser atherectomy is not as common as the prior devices discussed. Through absorption of energy within the atheroma, photothermal ablation is achieved via laser atherectomy which results in fragments less than 10μm in diameter. However, laser atherectomy may have lower success rates in severely calcified lesions and are best suited for lesions with moderate calcification. In severely calcified lesions, laser atherectomy can be first performed to achieve sufficient plaque modification to allow for microcatheter advancement. The guide wire can be exchanged for an atherectomy wire to allow for subsequent further plaque modification via rotational or orbital atherectomy. (4). The downside of laser atherectomy is that it is not readily available in most centres.
Conclusion
Emerging novel devices in recent years has greatly expanded the armamentarium for treatment of heavily calcified lesions. The challenge is for the operator to familiarize himself with the strengths and limitations of each of the devices so as to adopt the best individualized approach in treating the target lesion concerned.
References
- Mintz GS, Popma JJ, Pichard AD, et al. Patterns of calcification in coronary artery disease. A statistical analysis of intravascular ultrasound and coronary angiography in 1155 lesions. Circulation 1995;91:1959e1965.
- Généreux P, Madhavan MV, Mintz GS, et al. Ischemic outcomes after coronary intervention of calcified vessels in acute coronary syndromes. Pooled analysis from the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) and ACUITY (Acute Catheterization and Urgent Intervention Triage Strategy) TRIALS. J Am Coll Cardiol. 2014;63(18):1845-54.
- Sharma SK, Bolduan RW, Patel MR, et al. Impact of calcification on percutaneous coronary intervention: MACE-Trial 1-year results. Catheter Cardiovasc Interv. 2019;94(2):187-194.
- Kassimis G, Raina T, Kontogiannis N, et al. How Should We Treat Heavily Calcified Coronary Artery Disease in Contemporary Practice? From Atherectomy to Intravascular Lithotripsy. Cardiovasc Revasc Med. 2019;20(12):1172-1183.
- Barbato E, Carrié D, Dardas P, et al. European expert consensus on rotational atherectomy. EuroIntervention. 2015;11(1):30-6.
- Garcia-Lara J, Pinar E, Valdesuso R, et al. Percutaneous coronary intervention with rotational atherectomy for severely calcified unprotected left main: immediate and two-years follow-up results. Catheter Cardiovasc Interv. 2012;80(2):215-20.
- Lee M, Généreux P, Shlofmitz R, et al. Orbital atherectomy for treating de novo, severely calcified coronary lesions: 3-year results of the pivotal ORBIT II trial. Cardiovasc Revasc Med. 2017;18(4):261-264.
- De Silva K, Roy J, Webb I, et al. A Calcific, Undilatable Stenosis: Lithoplasty, a New Tool in the Box? JACC Cardiovasc Interv. 2017;10(3):304-306.
- Brinton TJ, Ali ZA, Hill JM, et al. Feasibility of Shockwave Coronary Intravascular Lithotripsy for the Treatment of Calcified Coronary Stenoses. Circulation. 2019;139(6):834-836.
